Current Research Topics
Ionic liquids • Tyler Parrack, Haoyun Sun
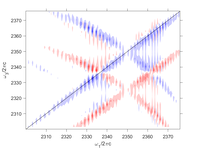
Ionic liquids are a promising platform for selective, high capacity, low energy-cost gas separation technologies. Progress in their development, however, is hindered by a lack of fundamental understanding of the structure and dynamics of these complex fluids as well as how they solvate molecular adsorbates. The rich variety of intermolecular interactions between the cations and anions cause unusual mesoscopic and macroscopic behaviors in these fluids. Ultrafast vibrational spectroscopy has the ability to interrogate molecular structure and picosecond intermolecular motions underlying these larger time- and length-scale phenomena. 2D-IR experiments will investigate both the bonding between cation and anion as well as the picosecond dynamics of CO2 and N2O in imidazolium ionic liquids. |
Ion Gels • Haoyun SunTo combat common downfalls in ionic liquid technology (high viscosity, cost, and industrial plant footprint), polymer and polymer composite materials have been investigated for use in CO2 capture. Several types of composite materials have been developed: supported ionic liquid membranes, polymerized ionic liquids, and ion gels.
The SGRLab has focused on the last type of composite material, which is made by mixing polymer chains with ionic liquid and then forming a solid. These materials exhibit different frequency dynamics and intramolecular vibrational relaxation from either of the parent materials. Understanding molecular-scale interactions of CO2 in complex composite systems offers insight into their potential usefulness as carbon capture materials. |
2D-IR Spectroscopy of Metal-binding Carboxylates • Sunayana Mitra
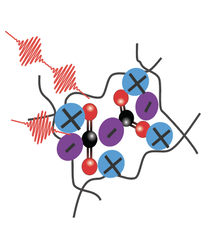
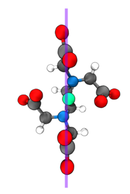
In all forms of life, proteins chelate metal ions to perform myriad functions. The ions may play a structural role (e.g. zinc finger proteins), a catalytic role (e.g. Fe in cytochromes), or a dynamic role (e.g. passing K+ ions through a membrane). Attacking the problem of carboxylate metal-binding with linear IR spectroscopy, two-dimensional infrared spectroscopy, and computational chemistry provides insights on the preference of Ca2+ binding over Mg2+ ion in cellular signaling. The projects final objective is to develop new methods to understand the coordination and geometry of different metals in metal-binding proteins and how that impact protein conformation.
In large part, this lack of understanding is due to a lack of experimental tools with the appropriate temporal- and spatial-resolution. Ultrafast vibrational spectroscopy addresses exactly these needs. Its time-resolution is sub-picosecond and can give residue specific information. The overarching goal of this project is to develop ultrafast vibrational spectroscopy as a general tool to interrogate the inner-sphere coordination of polypeptides with biologically important metal cations. |
2D Rovibrational Spectroscopy • Kai Gronborg, Sydney Giles2D-IR can also be used to obtain information about angular momentum dynamics in addition to vibrational frequency fluctuations. This technique can reveal insights into the shifts in magnitude and orientation of angular momentum. In this project, we study the angular momentum dynamics of CO2 in the gas phase. We utilize both linearly and circularly polarized light to investigate these effects.
In order to understand the complex nature of these spectra, we've developed a simulation based on quantum mechanical concepts propagated via perturbation theory. Statistical mechanics is then used to simulate angular momentum transfer between molecules due to collisions during population times. This helps to better understand intra- and intermolecular energy transfer and relaxation in the gaseous phase. |
Past Research Topics
Hydride transfers from borohydride • Clinton Johnson, Molly Wagner
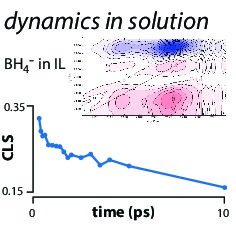
BH4- is a strong chemical reductant. Using ultrafast vibrational spectroscopy, we are probing how solvation controls the chemistry. We are interested in establishing both the dynamics at equilibrium (using 2D-IR spectroscopy) and the kinetics of reaction after photoexcitation.
|
Ion-Concentration Jump Experiment • Samrat Dutta
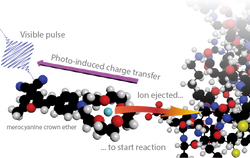
Ion uptake in EF hand is too fast for stopped flow experiments and single-molecule spectroscopy to time-resolve.
None of the standard time-domain techniques can break into the sub-microsecond regime, which is important to resolve elementary kinetic steps of the Ca2+ induced conformational change. A "caged-calcium" compound will be developed to create a photoinduced change in the ion concentration on a sub-picosecond timescale. This ion-concentration jump experiment will probe the reorganization of synthetic EF-hand polypeptides over the full range of timescales (picoseconds to microseconds) with residue specificity. We will learn about the elementary kinetic steps of ion binding, reorganization of the binding pocket, and the transmission of the binding signal to the global conformation of the domain.
None of the standard time-domain techniques can break into the sub-microsecond regime, which is important to resolve elementary kinetic steps of the Ca2+ induced conformational change. A "caged-calcium" compound will be developed to create a photoinduced change in the ion concentration on a sub-picosecond timescale. This ion-concentration jump experiment will probe the reorganization of synthetic EF-hand polypeptides over the full range of timescales (picoseconds to microseconds) with residue specificity. We will learn about the elementary kinetic steps of ion binding, reorganization of the binding pocket, and the transmission of the binding signal to the global conformation of the domain.
Collaborations
Electronic structure of carbon dioxide in ionic liquidsIn collaboration with Prof. Daniel Lambrecht's group (Florida Gulf Coast University, Chemistry), we are developing electronic structure methods to gain insight into the physical origin of the vibrational frequency shifts that CO2 reports. A decomposition scheme based on Absolutely Localized Molecular Orbitals (ALMOs) allows us to separate the many contributing effects – geometrical distortion, polarization, electrostatics, and charge transfer.
|
Molecular dynamics of carbon dioxide in ionic liquidsIn collaboration with Prof. Steve Corcelli's group (Notre Dame, Chemistry), we are using molecular dynamics simulations to assign and interpret the molecular motions that are reported in the 2D-IR spectroscopy. Insight into the molecular interactions between CO2 and ionic liquids is important for the design of future carbon capture systems.
|
Carbon dioxide reduction in green solventsIn collaboration with Prof. John Keith (Pitt, Chem. Eng.), we are investigating the role of solvents in controlling hydride transfers from strong chemical reductants like BH4-. The insight from this project is important for converting CO2 into a renewable fuel stock.
|